Introduction: Lenalidomide maintenance (LM) has shown progression-free (PFS) and overall survival (OS) benefit in clinical trials and is the recommended standard of care in patients with newly diagnosed multiple myeloma (MM) after high-dose melphalan and autologous stem cell transplantation (HDM-ASCT). In Denmark, LM has been approved for all patients treated with HDM-ASCT since June 2019 and is publicly funded. That LM was made available nationwide simultaneous across all regions creates a unique opportunity to study the real-world use pattern, efficacy, and adverse effects of LM in an unselected, population-based cohort without referral bias.
Methods: Patients with newly diagnosed MM treated with their first HDM-ASCT between June 2019 and March 2022 were included and followed until data cut-off in June 2023. To compare outcomes, a historical pre-LM cohort from the Danish MM Registry, consisting of MM patients treated with HDM-ASCT between June 2015 and June 2019, was used. Baseline clinical characteristics were acquired from the Danish MM Registry. To avoid the risk of guarantee-time bias, several strategies were applied. Early discontinuation of LM was studied using landmark analysis at 6 months after initiation of LM. For comparison with the pre-LM cohort, survival time was calculated from date of ASCT to progression or death for PFS and death of any cause for OS. Cox proportional hazards regression was used to calculate hazard ratios. Multivariate adjustment was made for age and sex as well as known risk factors for worse outcome: ISS and high-risk cytogenetics.
Results:
Lenalidomide maintenance cohort: Among 364 patients treated with HDM-ASCT after June 2019, 22.3% received consolidation therapy, and 3.7% underwent tandem HDM-ASCT. During follow-up, 297 patients (81.6%) initiated maintenance therapy, with 277 (76.1%) receiving LM. Overall, 145 patients (52.3%) discontinued LM most commonly due to toxicity 75 (51.7%), with fatigue (30.7%), cytopenia (25.3%), and neuropathy (17.3%) being the main reasons. During follow up, 119 (32.7%) patients had an event of progressive disease or death and 38 died. Only 1 death was related to Covid-19. To study the impact of early discontinuation of LM on clinical outcomes a 6-month landmark analysis was conducted. Early discontinuation did not negatively impact PFS, HR 0.92 (95% CI: 0.50, 1.70) or OS, HR 1.12 (0.25, 5.12). Results were similar in a 12-month landmark analysis.
Comparison to the pre-lenalidomide maintenance cohort:
A cohort of 364 multiple myeloma patients treated before the introduction of lenalidomide maintenance was used as comparison (pre-LM cohort). In that we take advantage of the randomness in being treated for MM in the years before and after the approval of lenalidomide. As the inclusion to either cohort was solely defined by the date of HDM-ASCT baseline characteristics were well balanced as expected with a median age of 61.1 vs 61.8 in the LM cohort and pre-LM cohort, respectively. By chance the dates of inclusion resulted in equal numbers of participants in both cohorts.
We confirmed that lenalidomide maintenance was not routinely used before the approval in June 2019 by reviewing the electronic records of all patients treated with HDM-ASCT between January and June 2019. Of these, only 3 (7%) received LM, and in each case, LM was initiated just prior to approval.
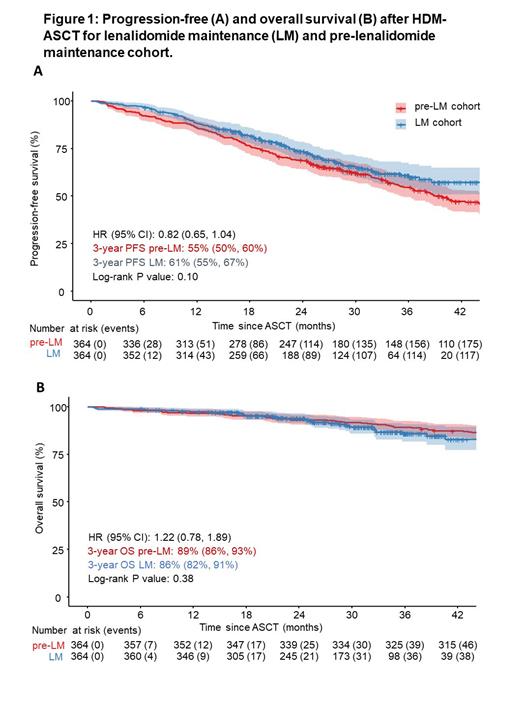
The LM cohort had similar PFS, HR 0.82 (0.65, 1.04), and OS HR 1.22 (0.78, 1.89) compared to the pre-LM cohort in unadjusted analysis, Figure 1. Results were similar after multivariate adjustment. The 3-year PFS and OS rates in the LM cohort were 61% (95 CI: 55%, 67%) and 86% (95 CI: 82%, 91%), respectively, while the pre-LM cohort had a 3-year PFS of 55% (50%, 60%) and a 3-year OS of 89% (86%, 93%).
Conclusion:
In this study encompassing all Danish patients with MM treated with HDM-ASCT since the introduction LM, we found that LM was initiated in approximately three out of four patients. One third of patients treated with LM discontinued this treatment within the first year, mainly due to toxicity. Early discontinuation of LM was not associated with worse clinical outcomes. When comparing post-transplantation PFS and OS in two cohorts of patients treated before and after the approval of LM, respectively, we found no differences.
Disclosures
Grønbæk:Kirsten Grønbæk received research support from Janssen and is on the advisory board of Nanexa and GSK.: Consultancy, Research Funding. Szabo:Janssen: Consultancy; Takeda: Consultancy, Research Funding; Sanofi: Consultancy; BMS: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal